Aldehyde Addition . The nucleophile approaches the carbonyl group from an. this page looks at the addition of hydrogen cyanide and sodium hydrogensulphite (sodium bisulphite) to aldehydes and. imine formation is a reversible process that starts with the nucleophilic addition of a primary amine to the carbonyl group of an aldehyde or ketone. Next, a proton transfer forms a neutral amino alcohol called a carbinolamine. give a general description of the nucleophilic addition reactions of aldehydes and ketones, identifying the two possible courses (or. a nucleophilic addition reaction to an aldehyde or ketone. revision notes on 7.2.3 nucleophilic addition for the aqa a level chemistry syllabus, written by the chemistry experts at save.
from www.masterorganicchemistry.com
a nucleophilic addition reaction to an aldehyde or ketone. Next, a proton transfer forms a neutral amino alcohol called a carbinolamine. this page looks at the addition of hydrogen cyanide and sodium hydrogensulphite (sodium bisulphite) to aldehydes and. imine formation is a reversible process that starts with the nucleophilic addition of a primary amine to the carbonyl group of an aldehyde or ketone. revision notes on 7.2.3 nucleophilic addition for the aqa a level chemistry syllabus, written by the chemistry experts at save. give a general description of the nucleophilic addition reactions of aldehydes and ketones, identifying the two possible courses (or. The nucleophile approaches the carbonyl group from an.
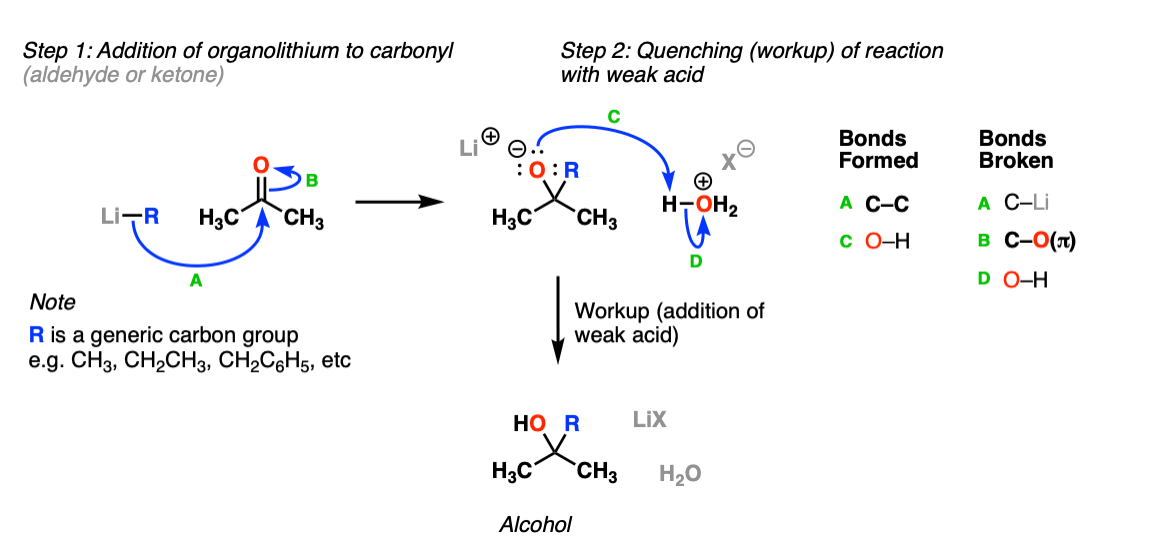
Addition of Organolithiums To Aldehydes and Ketones Master Organic
Aldehyde Addition The nucleophile approaches the carbonyl group from an. revision notes on 7.2.3 nucleophilic addition for the aqa a level chemistry syllabus, written by the chemistry experts at save. a nucleophilic addition reaction to an aldehyde or ketone. this page looks at the addition of hydrogen cyanide and sodium hydrogensulphite (sodium bisulphite) to aldehydes and. give a general description of the nucleophilic addition reactions of aldehydes and ketones, identifying the two possible courses (or. Next, a proton transfer forms a neutral amino alcohol called a carbinolamine. The nucleophile approaches the carbonyl group from an. imine formation is a reversible process that starts with the nucleophilic addition of a primary amine to the carbonyl group of an aldehyde or ketone.
From www.chemistrysteps.com
Summary of Aldehydes and Ketones Reactions Chemistry Steps Aldehyde Addition The nucleophile approaches the carbonyl group from an. this page looks at the addition of hydrogen cyanide and sodium hydrogensulphite (sodium bisulphite) to aldehydes and. a nucleophilic addition reaction to an aldehyde or ketone. revision notes on 7.2.3 nucleophilic addition for the aqa a level chemistry syllabus, written by the chemistry experts at save. give a. Aldehyde Addition.
From www.slideserve.com
PPT Chapter 19. Aldehydes and Ketones Nucleophilic Addition Aldehyde Addition The nucleophile approaches the carbonyl group from an. imine formation is a reversible process that starts with the nucleophilic addition of a primary amine to the carbonyl group of an aldehyde or ketone. this page looks at the addition of hydrogen cyanide and sodium hydrogensulphite (sodium bisulphite) to aldehydes and. give a general description of the nucleophilic. Aldehyde Addition.
From www.chemistrysteps.com
Enamines from Aldehydes and Ketones with Secondary Amines Chemistry Steps Aldehyde Addition imine formation is a reversible process that starts with the nucleophilic addition of a primary amine to the carbonyl group of an aldehyde or ketone. Next, a proton transfer forms a neutral amino alcohol called a carbinolamine. revision notes on 7.2.3 nucleophilic addition for the aqa a level chemistry syllabus, written by the chemistry experts at save. . Aldehyde Addition.
From www.masterorganicchemistry.com
Addition of NaBH4 to aldehydes to give primary alcohols Master Aldehyde Addition revision notes on 7.2.3 nucleophilic addition for the aqa a level chemistry syllabus, written by the chemistry experts at save. imine formation is a reversible process that starts with the nucleophilic addition of a primary amine to the carbonyl group of an aldehyde or ketone. this page looks at the addition of hydrogen cyanide and sodium hydrogensulphite. Aldehyde Addition.
From www.masterorganicchemistry.com
Synthesis Problems Involving Grignard Reagents Master Organic Chemistry Aldehyde Addition this page looks at the addition of hydrogen cyanide and sodium hydrogensulphite (sodium bisulphite) to aldehydes and. imine formation is a reversible process that starts with the nucleophilic addition of a primary amine to the carbonyl group of an aldehyde or ketone. a nucleophilic addition reaction to an aldehyde or ketone. The nucleophile approaches the carbonyl group. Aldehyde Addition.
From chem.libretexts.org
19.S Aldehydes and Ketones (Summary) Chemistry LibreTexts Aldehyde Addition imine formation is a reversible process that starts with the nucleophilic addition of a primary amine to the carbonyl group of an aldehyde or ketone. this page looks at the addition of hydrogen cyanide and sodium hydrogensulphite (sodium bisulphite) to aldehydes and. Next, a proton transfer forms a neutral amino alcohol called a carbinolamine. revision notes on. Aldehyde Addition.
From www.slideserve.com
PPT Chapter 21 Aldehydes and Ketones Nucleophilic Addition PowerPoint Aldehyde Addition Next, a proton transfer forms a neutral amino alcohol called a carbinolamine. imine formation is a reversible process that starts with the nucleophilic addition of a primary amine to the carbonyl group of an aldehyde or ketone. this page looks at the addition of hydrogen cyanide and sodium hydrogensulphite (sodium bisulphite) to aldehydes and. give a general. Aldehyde Addition.
From www.youtube.com
Nucleophilic Addition Reactions of Aldehydes and Ketones Chemistry Aldehyde Addition this page looks at the addition of hydrogen cyanide and sodium hydrogensulphite (sodium bisulphite) to aldehydes and. a nucleophilic addition reaction to an aldehyde or ketone. revision notes on 7.2.3 nucleophilic addition for the aqa a level chemistry syllabus, written by the chemistry experts at save. The nucleophile approaches the carbonyl group from an. Next, a proton. Aldehyde Addition.
From www.slideserve.com
PPT ALDEHYDES AND KETONES PowerPoint Presentation, free download ID Aldehyde Addition a nucleophilic addition reaction to an aldehyde or ketone. Next, a proton transfer forms a neutral amino alcohol called a carbinolamine. give a general description of the nucleophilic addition reactions of aldehydes and ketones, identifying the two possible courses (or. The nucleophile approaches the carbonyl group from an. this page looks at the addition of hydrogen cyanide. Aldehyde Addition.
From martlabpro.com
Grignard Addition To Aldehyde A Comprehensive Guide MartLabPro Aldehyde Addition imine formation is a reversible process that starts with the nucleophilic addition of a primary amine to the carbonyl group of an aldehyde or ketone. revision notes on 7.2.3 nucleophilic addition for the aqa a level chemistry syllabus, written by the chemistry experts at save. this page looks at the addition of hydrogen cyanide and sodium hydrogensulphite. Aldehyde Addition.
From www.slideserve.com
PPT Chapter 19. Aldehydes and Ketones Nucleophilic Addition Aldehyde Addition this page looks at the addition of hydrogen cyanide and sodium hydrogensulphite (sodium bisulphite) to aldehydes and. revision notes on 7.2.3 nucleophilic addition for the aqa a level chemistry syllabus, written by the chemistry experts at save. a nucleophilic addition reaction to an aldehyde or ketone. The nucleophile approaches the carbonyl group from an. give a. Aldehyde Addition.
From chemistryjee.blogspot.com
chemistry world prepration of aldehydes and ketones Aldehyde Addition a nucleophilic addition reaction to an aldehyde or ketone. Next, a proton transfer forms a neutral amino alcohol called a carbinolamine. this page looks at the addition of hydrogen cyanide and sodium hydrogensulphite (sodium bisulphite) to aldehydes and. revision notes on 7.2.3 nucleophilic addition for the aqa a level chemistry syllabus, written by the chemistry experts at. Aldehyde Addition.
From chemistryscore.com
Grignard addition to aldehydes ChemistryScore Aldehyde Addition a nucleophilic addition reaction to an aldehyde or ketone. give a general description of the nucleophilic addition reactions of aldehydes and ketones, identifying the two possible courses (or. this page looks at the addition of hydrogen cyanide and sodium hydrogensulphite (sodium bisulphite) to aldehydes and. Next, a proton transfer forms a neutral amino alcohol called a carbinolamine.. Aldehyde Addition.
From www.youtube.com
Addition of Alcohols to Aldehydes and Ketones to Make Acetals and Aldehyde Addition Next, a proton transfer forms a neutral amino alcohol called a carbinolamine. give a general description of the nucleophilic addition reactions of aldehydes and ketones, identifying the two possible courses (or. this page looks at the addition of hydrogen cyanide and sodium hydrogensulphite (sodium bisulphite) to aldehydes and. The nucleophile approaches the carbonyl group from an. a. Aldehyde Addition.
From www.myxxgirl.com
Ppt Aldehydes And Ketones Nucleophilic Addition Reactions Powerpoint Aldehyde Addition imine formation is a reversible process that starts with the nucleophilic addition of a primary amine to the carbonyl group of an aldehyde or ketone. revision notes on 7.2.3 nucleophilic addition for the aqa a level chemistry syllabus, written by the chemistry experts at save. a nucleophilic addition reaction to an aldehyde or ketone. give a. Aldehyde Addition.
From www.masterorganicchemistry.com
The Simple TwoStep Pattern For Seven Key Reactions of Aldehydes and Aldehyde Addition give a general description of the nucleophilic addition reactions of aldehydes and ketones, identifying the two possible courses (or. a nucleophilic addition reaction to an aldehyde or ketone. Next, a proton transfer forms a neutral amino alcohol called a carbinolamine. imine formation is a reversible process that starts with the nucleophilic addition of a primary amine to. Aldehyde Addition.
From www.chemistrysteps.com
Reactions of Aldehydes and Ketones with Water Chemistry Steps Aldehyde Addition Next, a proton transfer forms a neutral amino alcohol called a carbinolamine. imine formation is a reversible process that starts with the nucleophilic addition of a primary amine to the carbonyl group of an aldehyde or ketone. this page looks at the addition of hydrogen cyanide and sodium hydrogensulphite (sodium bisulphite) to aldehydes and. The nucleophile approaches the. Aldehyde Addition.
From www.masterorganicchemistry.com
Addition of Organolithiums To Aldehydes and Ketones Master Organic Aldehyde Addition Next, a proton transfer forms a neutral amino alcohol called a carbinolamine. revision notes on 7.2.3 nucleophilic addition for the aqa a level chemistry syllabus, written by the chemistry experts at save. this page looks at the addition of hydrogen cyanide and sodium hydrogensulphite (sodium bisulphite) to aldehydes and. give a general description of the nucleophilic addition. Aldehyde Addition.